










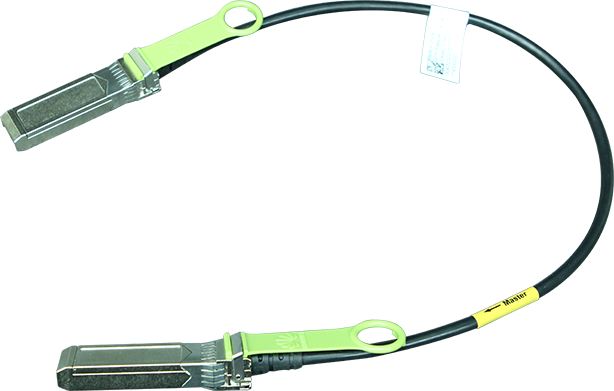







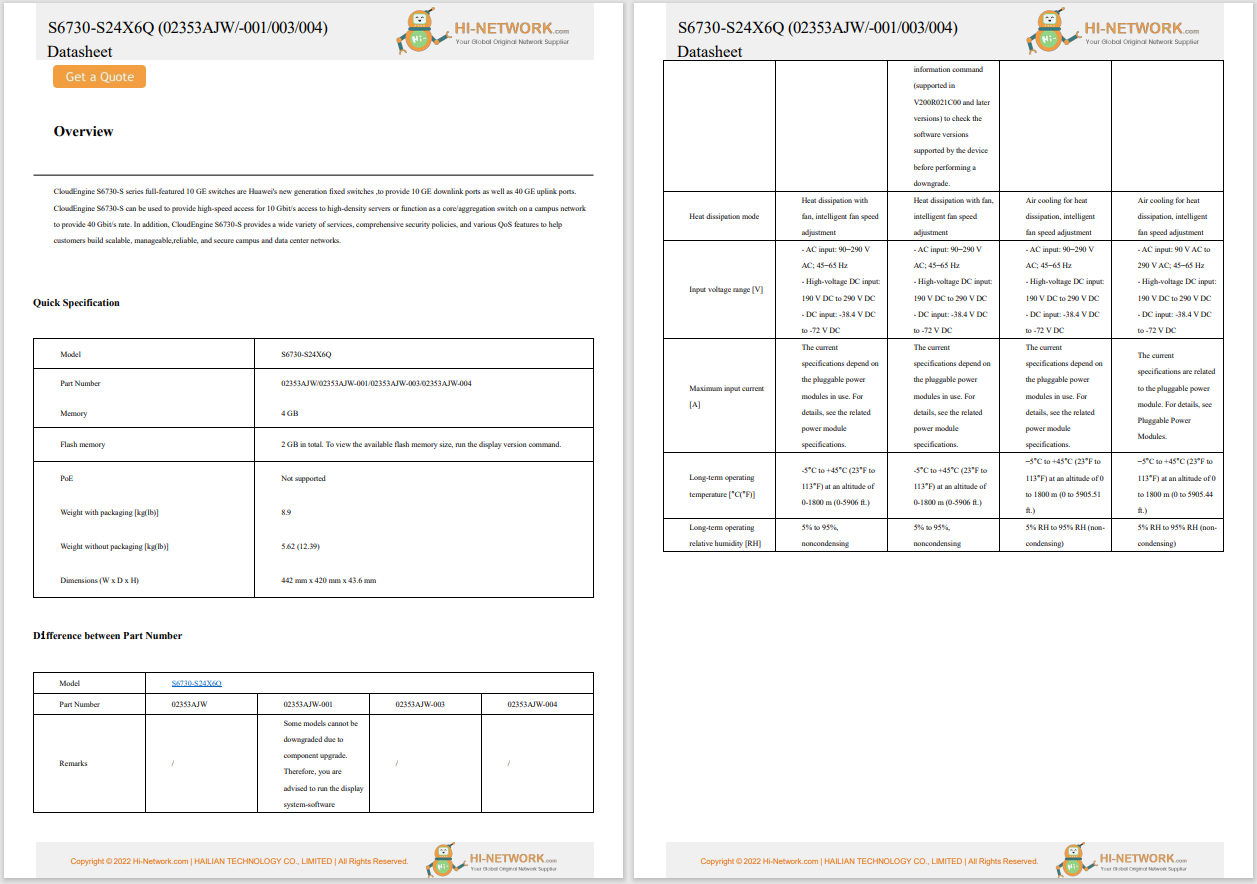
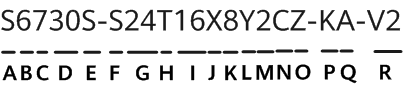












On 27 October 2022, the United States Food and Drug Administration's (FDA) Digital Health Center of Excellence (DHCoE) published a paper, titled 'Spotlight: Digital Health Regulatory Science Opportunities', to highlight important areas for consideration, identified by internal and external stakeholders of the FDA. Progress in digital health occurs quickly, and new areas of interest are likely to emerge, which prompts new regulatory science research focus areas throughout the ecosystem. The areas highlighted are: advancing patient engagement; leveraging connectivity; and improving health care through software.
 Etiquetas calientes:
Digital Health: Technology applications (en inglés)
Etiquetas calientes:
Digital Health: Technology applications (en inglés)
Regístrepor correo electrónico ahora para acciones semanales de promoción
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel en Hong Kong: 00852 66181601
Correo electrónico: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português