







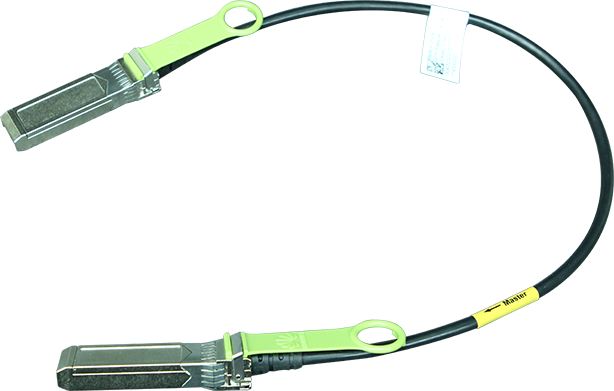







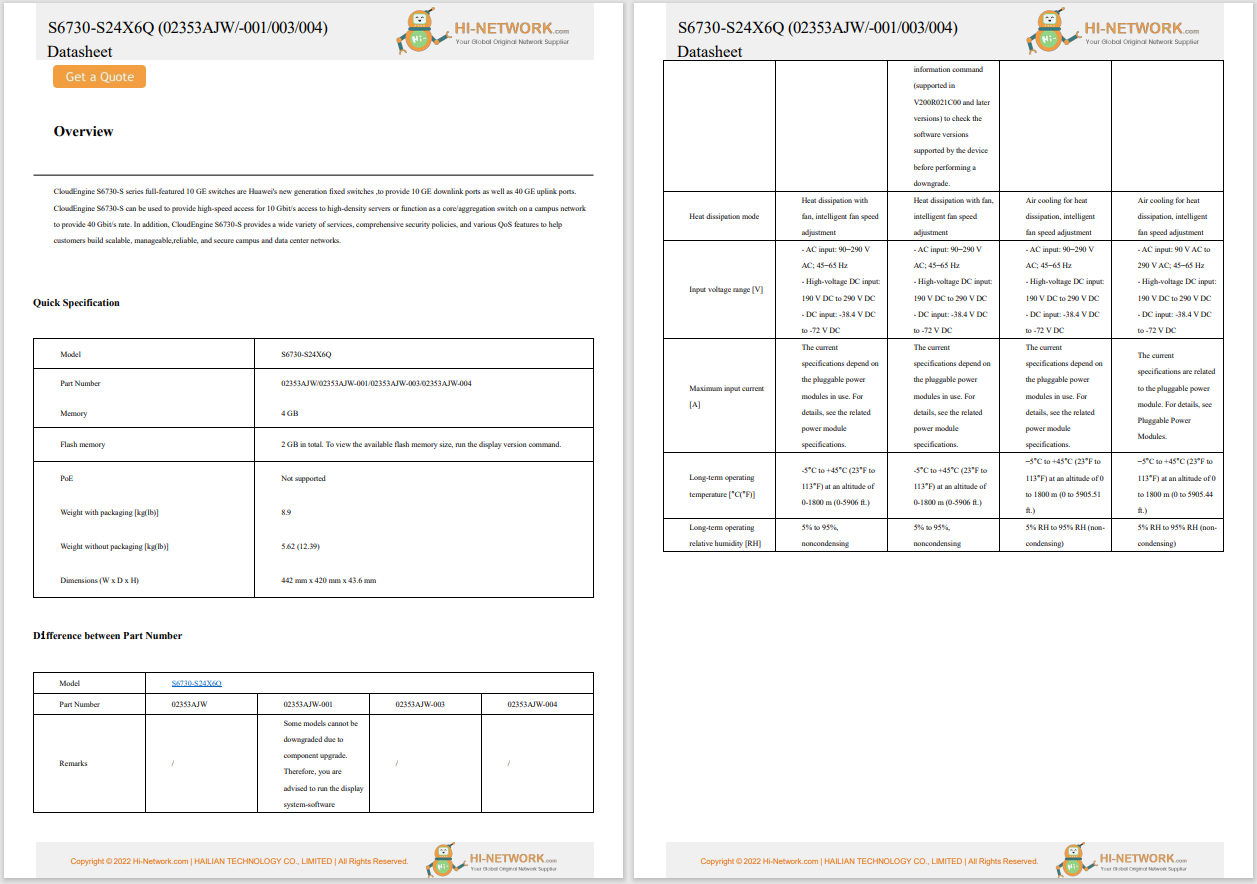
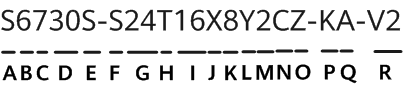















The U.S. Food and Drug Administration's (FDA's) Center for Devices and Radiological Health (CDRH) published best practices for communicating cybersecurity vulnerabilities to patients. The document provides information for the FDA, federal partners, and industry stakeholders to inform patients and the public about cybersecurity vulnerabilities. The paper highlights the following points: (1) make the content accessible (timely, relevant, simple, and readable) for people to read and understand; (2) discuss risks and benefits; (3) acknowledge and explain the unknown; (4) make it easy for patients to find and use information in online searches, and mobile devices.
 Etiquetas calientes:
Seguridad de red
Protección de los consumidores
Internet de las cosas
Etiquetas calientes:
Seguridad de red
Protección de los consumidores
Internet de las cosas
Regístrepor correo electrónico ahora para acciones semanales de promoción
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel en Hong Kong: 00852 66181601
Correo electrónico: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português