




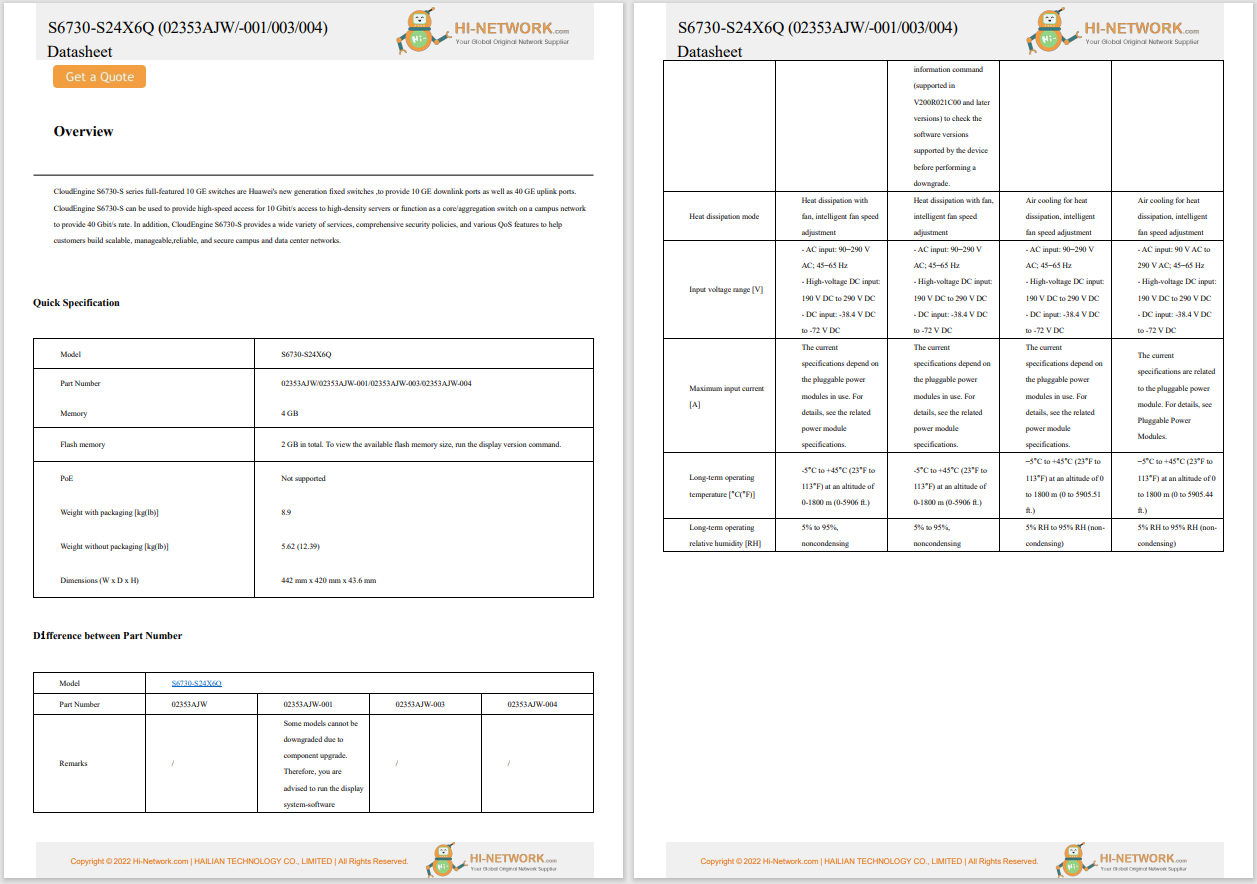





















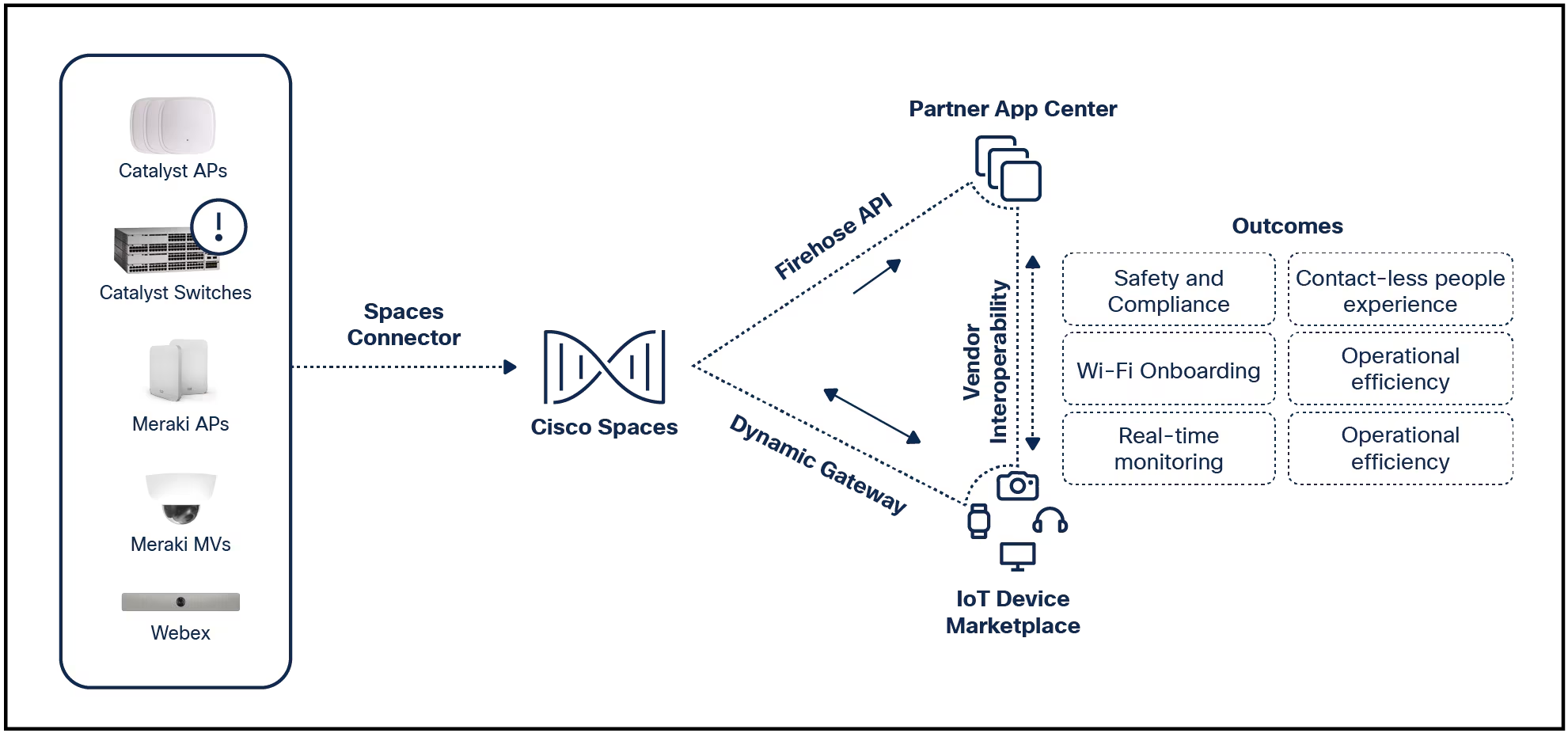

The US Food and Drug Administration (FDA) issued a warning about cybersecurity risks affecting Medtronic insulin pumps. According to the warning, the vulnerabilities enable unauthorised persons to connect wirelessly to the pump and to alter the pump's settings, leading to changes in blood sugar levels and possibly diabetic ketoacidosis. The company recalled the faulty pumps and provided alternative pumps to patients. So far, the FDA has not received any reports of patient harm related to the potential cybersecurity risks.
 Etiquetas calientes:
Seguridad de red
Internet de las cosas
Etiquetas calientes:
Seguridad de red
Internet de las cosas
Regístrepor correo electrónico ahora para acciones semanales de promoción
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel en Hong Kong: 00852 66181601
Correo electrónico: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português